Newsletter 2023
Newsletter November 2023: Label it the same way - standardised anaesthetic syringe label & prefilled syringe
Dr Kean Seng Cheah
Drug preparation & administration to the patient is the ‘bread & butter’ in anaesthesia practice. The complexity of this process, from drawing out medication from the ampoule into the syringe, checking, labelling and later from the syringe to the patient, has potential errors in various stages, so the human factor in medication safety is always on the brink of jeopardy. However, the human factor is not to be blamed entirely, as the inevitable human behaviour is affected by systemic failure. The systemic failure discussed here is the failure to standardise syringe labelling. A systematic and standardised step-based approach for thoroughness has been in practice for decades, such as anaesthetic machine check (1990)¹, and safe surgery saves lives check (2008)² and stop before you block (2011)³.
Every anaesthetist has a passion for arranging and labelling syringes in the workstation. This is usually influenced by the workplace’s ‘standard of practice’. When the anaesthetist moves on to a new place, this ‘standard’ may clash, and differences often result in unfamiliarity among peers. This unfamiliarity is always the ignition of a medication error event.
The primary drive of the ISO standard in 2008 (ISO 26825:2008) is to implement standardisation of the user-applied label for syringes in anaesthesia, including the colours, design and performance⁴. This, by far, is the reference standard as international guidelines. The Association of Anaesthetists recently revised the drug label (BS ISO 26825:2020)⁵ after the 2020 edition. Some changes have been made to improve the colours and design coding of high-risk drugs (Suxamethonium, Adrenaline, Benzodiazepines) and the introduction of tall man lettering labels. This is a good initiative by the association, but systemic failure still hinders medication safety. Consistency and standardisation can minimise misunderstanding and error. This also applies to as minuscule as sticking the label in a standardised way.
Similar to the process of induction & intubation, drug preparation in an anaesthetic room should be taken seriously by all theatre staff. The time when the anaesthetist preparing the drugs should be kept from anyone to avoid distraction. This should be made aware by all theatre staff, including the surgeon.
‘Label before’ or ‘label after’ filling?
Some anaesthetists advocate labelling before filling the syringe. While unlabelled syringes are known to be problematic, labelling on an empty syringe beforehand is never correct and is potentially more harmful. The following section will mention one example of how pre-labelling an empty syringe could compromise safety. The European Board of Anaesthesiology recommendations on safe medication practice recommend that labels should never be put on empty syringes. The syringe should be immediately labelled after filling before leaving the operator’s hand⁶.
Label horizontal, longitudinal or both?
Next, how should we stick the label on the syringe? The Association of Anaesthetists suggest sticking one label longitudinally (at least) so that the syringe can be read easily on the work surface⁷ (Never horizontal or diagonal). Horizontal labelling has disadvantages as it is obscure, and errors tend to occur during critical times, especially if both share the same Pantone matching system (PMS). For example, Atropine and Glycopyrrolate (pic). Although both are in the same class with the same PMS, it is still considered an error’ if given wrongly by mistake. This is less likely in longitudinal labelling. Longitudinal labelling alone is acceptable by the majority, but this is still not a complete safeguard, as there is still a small chance of error. The longitudinal label is easily ‘out of sight’ by slightly rotating the syringe; this can be easily mistaken as the ‘unlabelled’ syringe (b). This argument is backed up by an actual near-event incident when 2 anaesthetists prepare the anaesthetic drugs on the work surface. The first anaesthetist labelled the syringe ‘fentanyl’ longitudinally on the empty syringe(a), but the second anaesthetist did not notice the longitudinal label placed on an empty syringe (b), so it was used for a different drug and labelled it horizontally ‘Dexamethasone’ (c&d). This is the perfect example of how a lack of standardisation causes confusion & misunderstanding. Fortunately, this error was noticed immediately before the actual syringe swap occurred. To eliminate the slightest chance of error, it should be placed BOTH longitudinally and horizontally on the syringe, or the least longitudinally, with a ‘label after filling’ approach.
The pre-printed label is ‘out of stock.’
It is now common to find ISO pre-printed labels in a roll for dispensers in anaesthetic rooms, but efforts are needed to ensure that the stock is available in all theatres, all the time. One problem commonly faced by anaesthetists is when the particular drug label runs ‘out of stock’, an alternative way will be sought for drug labelling, such as:
- Handwritten the name using a different class label
- Handwritten the name on a ‘sodium chloride/saline’ label
There is no recommendation from the association or national guidelines to advise on what is the best practice if a pre-printed label is not available, but a safer practice would be using a blank (white) rather than a coloured label and keeping the drug in a separate tray to avoid error or given by mistake. The solution to this situation is still open for discussion & suggestions.
Prefilled syringe
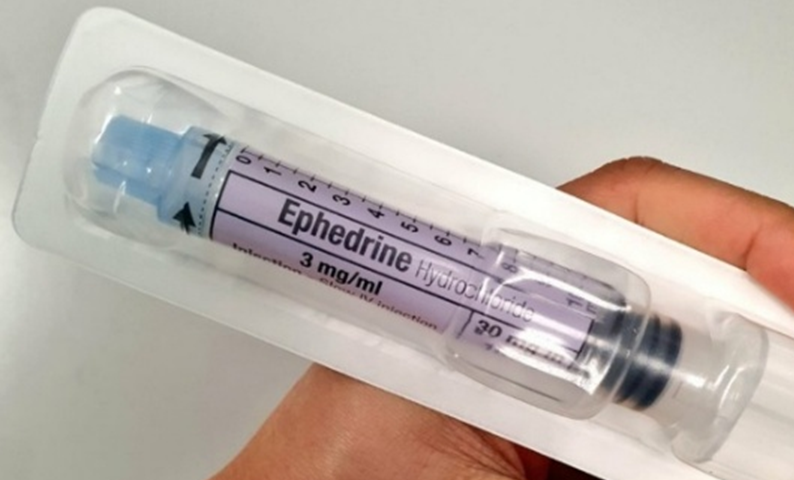
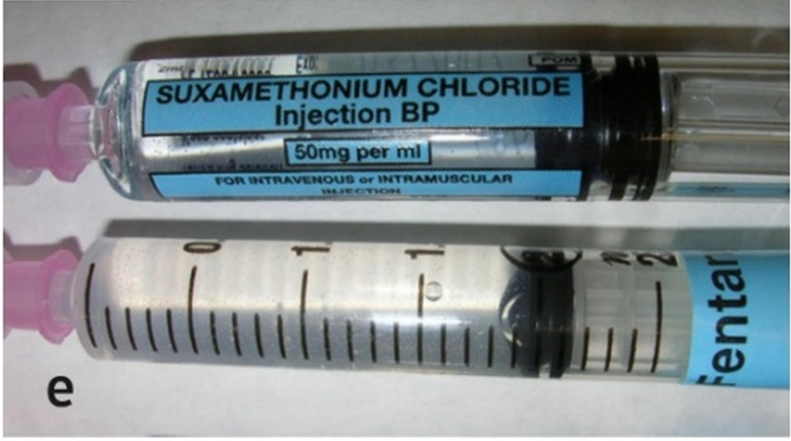
Nevertheless, there have been cases of near-missed medication with prefilled syringes reported, mainly due to confusion from the packaging and colour⁸ (e.g., The similar blue colour of Fentanyl for use in the high-risk drug Suxamethonium). The colours, packaging and designs of prefilled syringes should ideally be consistent with the ISO standard, although there is still not legal obligation for the manufacturer to follow the ISO. Hopefully, there will be positive changes in the future.
References
- Association of Anaesthetists of Great Britain and Ireland. London: Association of Anaesthetists of Great Britain and Ireland, 1990.
- https://www.who.int/teams/integrated-health-services/patient-safety/research/safe-surgery
- French J, Bedforth J, Townsley P. Stop Before You Block Campaign. https://www.rcoa.ac.uk/sites/default/files/documents/2020-08
- International Organization for Standardization. Anaesthetic and respiratory equipment.1st ed. Geneva: ISO, 2008. ISO 26825:2008(E).
- Association of Anaesthetists guideline. https://anaesthetists.org/Home/Resources-publications/Guidelines/Syringe-labelling-in-anaesthesia-and-critical-care-areas-review-2022
- Whitaker D, Brattebø G, Trenkler S et al. Eur J Anaesthesiol. 2017;34:4.
- David Whitaker. Association of Anaesthetists. News Digital Feb 2021.
- Patel BV. BJA 2006;96: 270.