Congress Newsletter 2024
More standardised – and more individualised: How AI-guided decision support can enhance goal-directed fluid therapy
How do we tailor intraoperative fluid therapy to optimise haemodynamics for each patient, while providing this individualised care in a standardised way? These questions lie at the heart of goal-directed therapy (GDT), a strategy recognised as best practice for high-risk surgical patients, but currently underutilised due to implementation difficulties.1 Recent work to address these difficulties has yielded a new technology – a fluid management software system that uses machine learning to provide decision support to clinicians. Alexandre Joosten, Associate Professor of Anaesthesiology at UCLA, California, USA has devoted much research to developing and testing this technology in clinical studies, and here he gives his insights on how this new software could help improve patient outcomes.
Dr. Joosten began by reviewing the rationale for GDT, first recapping why these protocols utilise advanced haemodynamic variables such as stroke volume, or dynamic variables indicating fluid responsiveness such as stroke volume variation or pulse pressure variation. These variables, he explained, provide a better indication of fluid responsiveness than static parameters such as heart rate and blood pressure. Determining fluid responsiveness is crucial to ensure adequate intravascular blood volume whilst avoiding fluid overload and thus postoperative complications. Only about half of haemodynamically unstable patients are fluid responsive; for the other half, fluid loading will not increase stroke volume and could cause more harm than good.2
“We need to continually assess these advanced variables in our protocols,” Dr. Joosten emphasised. “This information is key for optimising haemodynamics during high-risk surgery.”
Monitoring is only half of the equation, he continued, as we must then respond to the haemodynamic data appropriately with a clearly defined haemodynamic strategy. This is why GDT consists of step-by-step protocols in which clinicians follow treatment algorithms to determine whether a fluid bolus should be given. This standardisation is much-needed in fluid management, as variability between different care providers and situations can be significant – one study found that the top predictor of infusion volume during abdominal surgery was the clinical personnel.3
Overall, Dr. Joosten observed that evidence for the clinical benefits of GDT is compelling, but adoption of and adherence to these protocols remains low. “Many trials have indicated that perioperative GDT may reduce postoperative complications following high-risk surgery, and this strategy has been recommended by several anaesthesia societies across the world,” he noted. “However, use of GDT remains limited, and a major reason for this is that the complexity and variety of the protocols can make them difficult to implement at the bedside.”
To demonstrate this low utilisation, Dr. Joosten quoted two surveys of anaesthesiologists, one conducted in 20114 and the second in 2021.5 Both found that only about a third of respondents used cardiac output monitoring for patients undergoing high-risk surgery. In the 2021 survey, standardised treatment protocols for haemodynamic management were used by only 23% of European anaesthesiologists, a figure even lower than the 30% in the 2011 survey.
“A decade after the first survey, we had not made any significant progress in terms of monitoring high-risk patients to guide fluid administration,” he reflected. “So there is a clear need to ease the implementation of GDT at the bedside.”
As well as overcoming the practical barriers, there is a need to improve the effectiveness of GDT protocols. Illustrating this, Dr. Joosten highlighted a secondary analysis of the OPTIMISE trial, the largest multicentre study on GDT outcomes to date. The analysis revealed that just under 30% of fluid boluses initiated by the clinician resulted in an appropriate increase in stroke volume.6
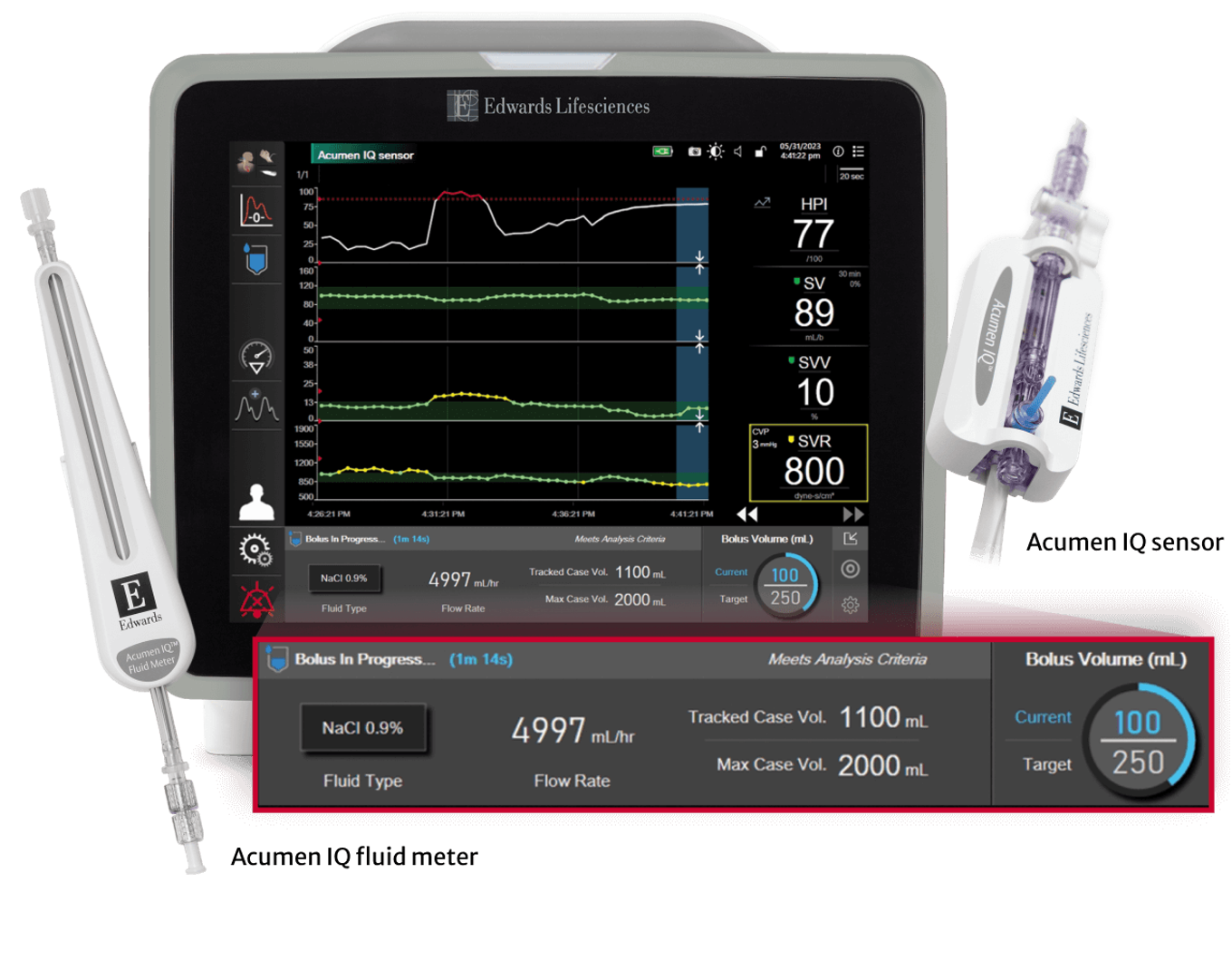
To address these two issues of effectiveness and ease of implementation, Dr. Joosten and his team have been investigating how to support clinical decision-making in GDT. In particular, they have focused on an AI-enhanced tool: Acumen™ Assisted Fluid Management (AFM™) software (Edwards Lifesciences, USA).
Acumen AFM™ software uses machine learning to predict fluid responsiveness for each patient, based on the patient’s haemodynamic data, and then uses these predictions to make individualised recommendations for fluid bolus administration. “The software has an elegant design: its predictions factor in population-level data as well as individual data from the current patient’s previous responses to fluid boluses. Firstly, the software can look back in its database, and take guidance from patients in its memory with the same kind of haemodynamics… and secondly it can learn from the current patient, so its predictions become more and more accurate and individualised over the course of the surgery,” explained Dr. Joosten.
Dr. Joosten and his team evaluated this software in a number of trials. The first compared Acumen AFM™ software management (46 patients) with manual GDT (a historical cohort of 38 controls) in major abdominal surgery. Results of this small study were encouraging: patients in the Acumen AFM™ software group spent more time in a preload-independent state, defined as a stroke volume variation under a threshold of 13%, than those in the control group (median 92% vs. 76%; p < 0.0005).7
A subsequent larger multicentre study enrolled 330 adults who underwent moderate- to high-risk noncardiac surgery; Acumen AFM™ software was used and clinicians were also free to initiate fluid boluses themselves. The primary outcome was the proportion of software-recommended boluses that achieved a desired increase in stroke volume compared to a 30% reference rate. This reference rate was exceeded twofold by the software, with 66% of boluses resulting in desired stroke volume increases. In comparison, 41% of clinician-initiated boluses had this effect (p < 0.0001). Further analysis revealed that the mean increase in stroke volume was higher after software-recommended than clinician-initiated boluses (14.2% vs. 8.3%; p < 0.0001).8
“We saw that boluses initiated by clinicians are less often effective to optimise stroke volume,” summarised Dr. Joosten. “This showed the value of the software recommendations.”
The latest study on Acumen AFM™ software to be published by Dr. Joosten and his team assessed this software in combination with a closed-loop system for vasopressor administration. In a randomised controlled trial of 38 patients undergoing abdominal or orthopaedic surgery, they compared this ‘computer-assisted GDT’ to manual GDT. The percentage of intraoperative case time a patient had hypotension (defined as a mean arterial pressure < 90% of their baseline) was only 1.2% in the computer-assisted group, as compared to 21.5% in the manual group (p < 0.001). Mean stroke volume index and cardiac index were also higher in the computer-assisted group (p < 0.001).9 “Computer-assisted GDT clearly outperformed standard practice,” observed Dr. Joosten.
As well as describing these three published studies, Dr. Joosten shared a glimpse of new results from two studies in press. “In one study, we looked at patients undergoing high-risk abdominal surgery, and found that those who had Acumen AFM™ software-guided GDT had higher sublingual microvascular flow than those who had manual GDT.”
“In the second, we looked at major liver surgery, and we found that arterial lactate at the end of surgery was lower with Acumen AFM™ software guidance than with a more restrictive fluid strategy,” he described. “We hope these studies will be published soon. Lastly, we are currently assessing the impact of Acumen AFM™ software on patient outcomes in a multicentre setting (NCT06011187).10 Results of this study will be available within the next two years.”
Considering all these studies, Dr. Joosten noted that the focus has generally been towards higher-risk surgeries, as this is the patient population likely to gain most benefit from this new technology. “Particularly for long and complex surgeries where you might expect some blood loss, or there may be abdominal compression that compromises venous return, there is a real need to optimise haemodynamics,” he said. “It is also in higher-risk surgeries that we tend to use an arterial line that we can link to an advanced haemodynamic monitoring device to provide the data for the decision support system.”
Acumen AFM™ software will be launched at Euroanaesthesia 2024, and Dr. Joosten is positive about how it will be received by clinicians and integrated into practice. He feels that while fully automated systems are a bigger step, decision support tools such as Acumen AFM™ software will be readily adopted. “I believe that in the coming years we’ll see increased use of these kinds of tools,” he predicted. “I think they will be appreciated by clinicians as they simply provide a recommendation, and the final decision rests with the anaesthesiologist.”
Overall, Dr. Joosten is optimistic that clinical decision support software will help improve patient outcomes. By lessening the clinical burden of GDT protocols, and improving adherence to them, this software will help clinicians to deliver individualised care in a standardised way. “These tools support us to deliver standardised healthcare. For us in anaesthesiology we’re looking to standardise how we optimise haemodynamics with fluid management, so we can improve patient outcomes.”
References
- Joosten, A., Alexander, B., Delaporte, A., Lilot, M., Rinehart, J., & Cannesson, M. (2015). Perioperative goal directed therapy using automated closed-loop fluid management: the future?. Anaesthesiology intensive therapy, 47(5), 517–523. https://doi.org/10.5603/AIT.a2015.0069
- Martin, G. S., Kaufman, D. A., Marik, P. E., Shapiro, N. I., Levett, D. Z. H., Whittle, J., MacLeod, D. B., Chappell, D., Lacey, J., Woodcock, T., Mitchell, K., Malbrain, M. L. N. G., Woodcock, T. M., Martin, D., Imray, C. H. E., Manning, M. W., Howe, H., Grocott, M. P. W., Mythen, M. G., Gan, T. J., & Miller, T. E. (2020). Perioperative Quality Initiative (POQI) consensus statement on fundamental concepts in perioperative fluid management: fluid responsiveness and venous capacitance. Perioperative medicine (London, England), 9, 12. https://doi.org/10.1186/s13741-020-00142-8
- Lilot, M., Ehrenfeld, J. M., Lee, C., Harrington, B., Cannesson, M., & Rinehart, J. (2015). Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: retrospective two-centre analysis. British journal of anaesthesia, 114(5), 767–776. https://doi.org/10.1093/bja/aeu452
- Cannesson, M., Pestel, G., Ricks, C., Hoeft, A., & Perel, A. (2011). Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Critical care (London, England), 15(4), R197. https://doi.org/10.1186/cc10364
- Moritz, F., Joosten, A., Scheeren, T..W.L., Duranteau, J., & Saugel, B. (2023). Haemodynamic monitoring and management in patients having noncardiac surgery: A survey among members of the European Society of Anaesthesiology and Intensive Care. European journal of anaesthesiology and intensive care, 2(1), e0017. https://doi.org/10.1097/EA9.0000000000000017
- MacDonald, N., Ahmad, T., Mohr, O., Kirk-Bayley, J., Moppett, I., Hinds, C. J., & Pearse, R. M. (2015). Dynamic preload markers to predict fluid responsiveness during and after major gastrointestinal surgery: an observational substudy of the OPTIMISE trial. British journal of anaesthesia, 114(4), 598–604. https://doi.org/10.1093/bja/aeu398
- Joosten, A., Hafiane, R., Pustetto, M., Van Obbergh, L., Quackels, T., Buggenhout, A., Vincent, J. L., Ickx, B., & Rinehart, J. (2019). Practical impact of a decision support for goal-directed fluid therapy on protocol adherence: a clinical implementation study in patients undergoing major abdominal surgery. Journal of clinical monitoring and computing, 33(1), 15–24. https://doi.org/10.1007/s10877-018-0156-x
- Maheshwari, K., Malhotra, G., Bao, X., Lahsaei, P., Hand, W. R., Fleming, N. W., Ramsingh, D., Treggiari, M. M., Sessler, D. I., Miller, T. E., & Assisted Fluid Management Study Team (2021). Assisted Fluid Management Software Guidance for Intraoperative Fluid Administration. Anesthesiology, 135(2), 273–283. https://doi.org/10.1097/ALN.
- Joosten, A., Rinehart, J., Van der Linden, P., Alexander, B., Penna, C., De Montblanc, J., Cannesson, M., Vincent, J. L., Vicaut, E., & Duranteau, J. (2021). Computer-assisted Individualized Hemodynamic Management Reduces Intraoperative Hypotension in Intermediate- and High-risk Surgery: A Randomized Controlled Trial. Anesthesiology, 135(2), 258–272. https://doi.org/10.1097/ALN.0000000000003807

- National Library of Medicine (U.S.). (2023, August – ). Assisted Fluid Management (AFM) System and Postoperative Outcome After High-risk Abdominal Surgery (PEFLA). Identifier NCT06011187. https://classic.clinicaltrials.gov/ct2/show/NCT06011187
Author
- Dr Alexandre Joosten, UCLA, Los Angeles.
This article was sponsored by Edwards Lifesciences.
For more information, please visit https://www.edwards.com/gb/healthcare-professionals/products-services/predictive-monitoring/acumen-afm
© 2024 Edwards Lifesciences Corporation. All rights reserved. PP–EU-8540 v1.0